Image Contest
In the Image Contest, PhD students present fascinating moments captured from their own scientific work. The images can be taken on a variety of scientific imaging devices. In the past years the contestants allowed a broader audience to appreciate the beauty of science on any level imaginable.
Here we present the entries of this years Image Contest.
Jens Aydin - Old proteins, new beauty
Orlando José Cortés Campo - A constellation of synaptic terminals in the mouse brain
Vinicius da Cruz Neris Gessner - Microtubes: The scaffolds and highways of eukaryotic cell life
Marietheres Evers - Meet Mickey Mouse at Disneyland - The PhD Budget Edition
Pia van gen Hassend - Things I have learned while searching for a single protein crystal
Sofia Heredia-Guerrero - THE DARK SIDE OF A FAILED TRANSFECTION
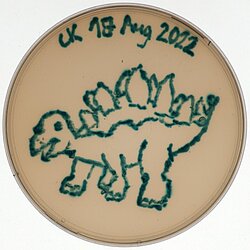
Charlotte Kamm - E.colisaurus
Manas Kshirsagar - Everyone feels a little differently!
Eduardo Merino Asumendi - Sweet home, ECM!
Marcel Rühling - View from a Spaceship on the Journey from Microcosm to Universe
Marie Schöl - Pregaming with my proteins
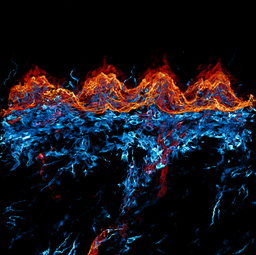
Anna Seubert - Fire and Ice
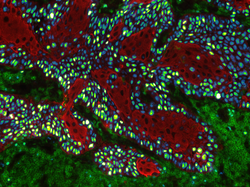
Lili Szabo - ‘Please, please don’t kill me…’
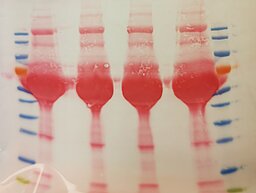
Jens Aydin
Title: Old proteins, new beauty
Silver nitrate is used to stain proteins, for example inside SDS gels. Silver ions bind to certain protein side chains and can be reduced to metallic silver to visualize the proteins inside the gel. Here, this was used compare extraction protocols for RNA-binding proteins. You can still see some patterns and different intensities between lanes in the gel…
After almost being forgotten in a lab drawer, the gel came back to light and revealed it had dried and shrunk resulting in a beautiful pattern of cracks. It did not fail to amaze the whole lab so I decided more people should be able to enjoy it!
Orlando José Cortés Campo
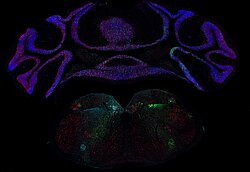
Title: A constellation of synaptic terminals in the mouse brain
A fluorescence microscope image capturing a coronal brain section from a Cre-driver mouse which was injected into the right and left dorsal vagal complex (DVC) in the medulla oblongata, with two different adeno-associated viruses (AAV) carrying the DNA of a green fluorescent protein (GFP) and a polypeptide protein tag (Myc-tag) fused to the synaptic vesicle protein synaptophysin.
The image shows in green and turquoise the signal detected from the GFP and Myc-tag present in the axonal terminals of vesicular glutamate transporter 2 positive neurons (Vglut2+) located in the DVC. The DVC which has been related to the cardiac interoception, respiratory regulation, and gustatory functions, projects to a number of brain areas, including the cerebellum of the mouse brain, as it is depicted in the picture.
In red, neuronal nuclear marker (NeuN), labelling exclusively neurons. In blue, counterstaining with DAPI shows the DNA within the different cell types in the mouse brain, it was used to highlight anatomical structures in the observed brain section.
Vinicius da Cruz Neris Gessner
Title: Microtubes: The scaffolds and highways of eukaryotic cell life
The Image is composed of Microtubes from NIH 3T3 cells. The microtube ultrastructure was stained for β-tubulin and the secondary antibody was coupled with Alexa fluor 647. The image acquired was 63x oil immersion and through a super-resolution microscope technique (dStorm). The microtube is formed by the polymerization of α/β-tubulin dimers and gives structure, shape, mediates internal cellular transport, and many other important functions in a eukaryotic cell. In general, the microtubes have 50 nm in diameter and for this reason, their visualization is limited by the diffraction limit of conventional microscopes. The dStrom imaging technique goes below this limit and makes it possible to visualize with high-resolution power these fascinating cellular ultrastructures.
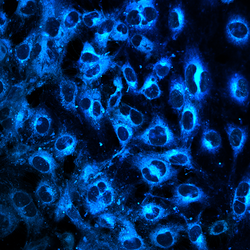
Marietheres Evers
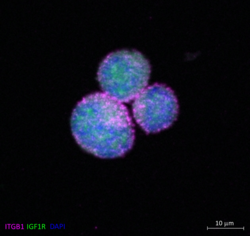
Title: Meet Mickey Mouse at Disneyland - The PhD Budget Edition
Integrin-b1 subunit (ITGB1) (pink) and the Insulin-like growth factor 1 receptor beta subunit (IGF1R) (green) were stained in single cells of the multiple myeloma cell line MM1S after stimulation with insulin-like growth factor 1 (IGF-1). While ITGB1 was localized in the membrane, IGF1R appeared to be localized in the cytoplasm. Nuclear staining was performed using DAPI (blue). A Zeiss Axio Observer-Z1 / 7 microscope with Plan-Apochromat 63x/1.40 Oil Ph3 M27 objective was used for this experiment.
Sofia Heredia-Guerrero
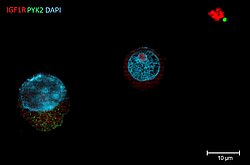
Title: THE DARK SIDE OF A FAILED TRANSFECTION
A Human Multiple Myeloma cell line (HMCL) was transfected with a mutant PYK2 construct via Sleeping Beauty System using electroporation. After stable transfection, cells were stained with DAPI for nucleus detection; as well as with antibodies against IGF1R (red) and PYK2 (green). This HMCL showed low survival and transfection efficiency (<10%). In this image we can observe a successfully transfected cell expressing mutant PYK2 (left), a cell with no PYK2 overexpression (middle) and debris of dead cells (right). Fluorescence signal was detected using Zeiss AxioObserver.Z1 (Plan Apochromat 63x/ 1,4 oil).
Charlotte Kamm
Title: E.colisaurus
My choice of colour for this scientific artwork was X-gal, an indoxyl glycoside consisting of galactose linked to a substituted indole, that was added to the LB agar plate. When cleaved by the lacZ – encoded ß-galactosidase present in E. coli, the indole is released resulting in bright blue staining of the bacterial cells.
With this experimental setup, I am testing different and partly novel CRISPR-Cas9 – based gene editors for their ability to disrupt lacZ expression resulting in the growth of white colonies on X-gal agar plates. In order to create my all-blue E.colisaurus, I transformed the bacteria with a deactivated Cas9 (dCas9) that shouldn’t facilitate lacZ disruption.
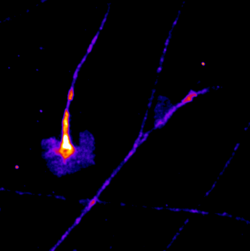
Manas Kshirsagar
Title: Everyone feels a little differently!
This is a timelapse image taken over 30 minutes The highly dynamic structures that are seen are called growth cones. These particular growth cones belong to Dorsal Root Ganglion sensory neurons of mouse embryonic origin, grown in a culture dish. The distal ends of the neurites from these neurons have growth cones and are influenced by an array of molecular signals that help them guide the axon growth to the synaptic target. These particular growth cones are made visible by the expression of a GFP tagged protein that is frequently localised on the cell membrane. The images were made on a Nikon TE2000 live imaging system, equipped with a Tokai Hit stage incubator.
Eduardo Merino Asumendi
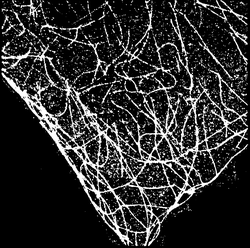
Title: Sweet home, ECM!
Extra cellular matrix (ECM) isolated from NIH3T3 fibroblasts. In tissues, fibroblasts are responsible for creating a spiderweb like structure called the ECM. The ECM, in organs, gives structural support “ a home” where several kinds of cells can harbour. In our lab we aim to functionalize the ECM with drugs, thus creating drug depots. In the left corner a control staining of the structural protein fribronectin is shown in red. In the middle part an enzymatically crosslinked peptide labelled with fluorescein as a cargo is shown in green. In the right corner the overlay of both fluorescence signals is displayed. This picture is relevant because it demonstrates how the ECM can be used to store medicaments by acting as a “home drug-storage” for the cells.
Marcel Rühling
Title: View from a Spaceship on the Journey from Microcosm to Universe
In this image human lung microvascular endothelial cells were stained by a FRET probe (Kappe et al., 2020, Chemistry 26, doi.org/10.1002/chem.202000133), mimicking sphingomyelin, a lipid component of eukaryotic membranes. The probe can be used to detect activity of a lipid-converting enzyme. It is integrated in cellular membranes, thereby visualizing cellular compartments. Like in the universe with a plethora of galaxies, a closer look might be worth, since many things can be discovered. Beside very bright structures that likely correspond to Golgi apparatus or the endoplasmic reticulum, faded mitochondria are meandering through the cells. If you look keen enough, you will discover gossamer filaments interconnecting individual cells.
Imaging was done on fixed samples with confocal laser scanning microscopy (Leica SP5). Similar as for Hubble Ultra Deep Field, long exposure times were needed to record the image in this quality. Thus, recording the image took ~45 min.
Anna Seubert
Title: Fire and Ice
This image shows a cross section of the murine dorsal tongue mucosa stained for a basal membrane marker (red, ITGb4) and a stromal marker (cyan, VIM). By displaying the interplay of those markers, we can describe the epithelial - stroma cell interaction. For example, the invasion of dendritic cells into the epithelial layers, here seen as cyan dendrites within the red coloured epithelium. Here we performed 3D imaging on a 200 µm thick frozen section using a 40x oil objective in a Zeiss LSM confocal microscope.
Lili Szabo
Title: ‘Please, please don’t kill me…’
In cancer, we can distinguish different immune phenotypes. There are cold tumors, where the immune cells are not represented or separated from the tumor cells, and there are hot tumors, where the immune cells are infiltrated into the tumor. This immunofluorescent picture represents a ‘cold tumor’ in the human dorsal tongue, where the immune cells (green) clearly separated from the tumor cells (red). Interestingly, this tongue tumor is highly proliferating based on the KI67 staining (blue, turning to yellow with the intensity), while we cannot see considerable proliferation among the immune cells. These facts make the sample suitable to model, and study the tumor cell – immune cell interactions using 3D organoid technology. The image was taken with EVOS M5000 Imaging System (Thermo Fisher Scientific).