Keynote Speaker
Prof. Alfredo Castello
Professor at University of Glasgow, Glasgow, UK
RNA Binding Proteins in Virus Infection
Alfredo Castello is Professor of Systems Virology at the MRC–University of Glasgow Centre for Virus Research. He began his career studying viral proteases and host gene expression during his PhD in Madrid, followed by a Marie Curie postdoctoral fellowship at EMBL Heidelberg, where he pioneered methods to identify RNA-binding proteins. In 2014, he established his lab at the University of Oxford with an MRC Career Development Award, focusing on the composition of viral ribonucleoproteins in infected cells. Since 2020, he has led an ERC-funded programme in Glasgow to dissect how cellular RNA-binding proteins regulate virus infection. His work combines virology, RNA biology, and proteomics to understand host–virus interactions at the systems level.
Keynote Talk (Oct. 8th, 9.30 am): Hidden Heroes: Nuclear RNA-binding proteins and their secret antiviral powers
RNA-binding proteins (RBPs) are emerging as central regulators of antiviral defence, yet their roles in infection remain largely unknown. In this talk, we unveil a hidden layer of host innate immunity orchestrated by nuclear RBPs, a class of proteins traditionally known for regulating gene expression and RNA metabolism. Using integrative proteomics, systems virology, and functional assays, we discovered that a substantial cohort of nuclear RBPs is relocalised during infection and exerts potent antiviral functions. These proteins form a distributed network that can restrict diverse RNA viruses, acting at multiple stages of the viral lifecycle. Our work reveals that the nucleus is not just a passive target of viral manipulation, but a proactive hub of host defence. This overlooked antiviral axis opens up new therapeutic avenues and reshapes how we think about innate immunity in the RNA–protein world.
Prof. Marina Kreutz
Professor at University Hospital Regensburg, Germany

Tumor and immune cell metabolism
Marina Kreutz studied Biology and perfomed her PhD thesis under the supervision of Prof. Reinhard Andreesen at the Albert-Ludwigs-Universität Freiburg (topic: Induction of human monocyte differentiation by Vitamin D3). She continued to work on this topic in Regensburg as a postdoc. In 1995, she moved to San Diego and worked as a research fellow at the University of California San Diego/UCSD (Prof. Dr. C. Glass). After her return to Germany, she graduated as “Privatdozent” (Habilitation) and finally became appointed as Professor for Molecular Oncology at the University Hospital Regensburg in 2012. Marina is also head of a Clinical Cooperation Group Immunometabolomics within the LIT-Leibniz Institute for Immunotherapy in Regensburg. Her main interest is the interplay between tumor-and immunometabolism. Currently the group focuses on different strategies to strengthen the response to immunotherapy in patients.
Keynote Talk (Oct. 8th, 11.00 am): The immunological Warburg effect- a metabolic checkpoint in the tumor environment
Accelerated glycolysis in ‘Warburg’ tumors leads to lactic acid accumulation and acidification of the tumor microenvironment. This results in lactate and proton uptake by tumor-infiltrating T cells, causing intracellular acidification, reduced respiration, and impaired T cell proliferation and function. However, the precise mechanisms of lactic acid-induced immunosuppression remain poorly understood.
Through transcriptome analysis and in vitro studies, we propose a novel mechanism of lactic acid-mediated immunosuppression in human T cells and identify potential strategies to restore T cell function in acidic environments. RNA-seq of T cells exposed to metabolic stress and lactic acid revealed upregulation of Solute Carrier Family members, particularly the cystine-glutamate antiporter SLC7A11, which is critical for glutathione synthesis and cellular protection against oxidative stress. Flow cytometry using DCFDA and CellRox demonstrated rapid cytoplasmic reactive oxygen species (ROS) induction following lactic acid exposure, without a corresponding increase in mitochondrial ROS. Notably, lactate alone did not trigger ROS production, while acidification partially replicated lactic acid’s effects. Treatment with N-acetylcysteine and other ROS scavengers diminished ROS production and partially restored T cell metabolism, proliferation, and function in vitro.
In vivo, LDHA/B knockout in B16 tumor cells reduced ROS levels in tumor-infiltrating immune cells, while human tumor-infiltrating immune cells in head and neck squamous cell carcinoma showed elevated ROS. These findings suggest that targeting ROS with antioxidants may offer a promising approach to counteract lactic acid-driven immunosuppression in the tumor microenvironment.
Dr. Katarina Wolf
Assistant Professor at Radboud University Medical Center, Nijmegen, Netherlands
Cell biology (Tumor metastasis - Cell migration - Cell-matrix interaction)
Dr. Katarina Wolf graduated in 2003 from the University of Würzburg, Germany, on the visualization of proteolytic cell-matrix interactions and dynamic cell patterning by using 3D extracellular-matrix-based cell-culture models as well as 3D connective tissue mouse models in intravital microscopy. She continued these studies as an assistant professor, in Nijmegen, The Netherlands. Her research interests are at the interface of cell biology, biophysics and cell mechanics, and modelling tumor dissemination-related processes. Ongoing work focuses on modulators of tumor cell invasion efficacy and plasticity, as well as the development of in vivo-inspired migration models in vitro. In particular, the role of nuclear stiffness-associated lamins during confined invasion and metastatic dissemination of fibrocarcoma or melanomas is being studied. The aim of this research is to bridge basic molecular to translational aspects of cancer disease.
Keynote Talk (Oct. 8th, 11.45 am): Impact of lamin-A/C expression modulation on cancer invasion and metastasis in vivo
Metastatic tumor cell invasion into 3D heterogenous interstitial tissues of network-, channel- or cleft-like architecture involves biomechanical adaptation of the cell including its nucleus to tissue geometry. In tumor cells nuclear A-type lamins contributing to the rigidity of the cell nucleus have been found to be deregulated, however, the biophysical roles of lamins in cancer cell migration and tumor cell survival within the heterogenous tissue structures in vivo remain unclear. Human fibrosarcoma and melanoma cell lines with different lamin-A/C levels were implanted into the dermis of living mice carrying a dorsal imaging window, and invasion was monitored by intravital multiphoton microscopy, followed by detection of circulating tumor cells and metastasis to distant organs. I will discuss on the progress of our work on the impact of A-type lamins on tumor cell integrity and metastatic dissemination in the living organism.
Prof. Hanna Engelke
Professor at University of Graz, Austria
Pharmaceutical Cell Biology (Mechanobiology - Optogenetics - Nanoparticles for Drug Delivery)
Hanna Engelke studied physics at Universität Bayreuth. After a PhD at LMU München in biophysics and a postdoc at UC Berkeley in mechanobiology, she worked as group leader at the department of chemistry of the LMU. From there, she moved to the University of Graz, where she currently holds a professorship for Pharmaceutical Cell Biology. Her lab focuses on mechanobiology, particularly the interactions between cells and the mechanics of the extracellular matrix. The lab develops tools to control matrix properties and intracellular signals in 3D cell culture models. With these tools, they investigate the influence of matrix and corresponding intracellular signaling on cell behavior. Another line of research in the lab is the development of nanoparticles for biomedical applications.
Keynote Talk (Oct. 8th, 1.30 pm): Cell-Matrix Interactions – from fibers to mechanotransduction
Cell behavior is strongly influenced by the chemical and mechanical properties of the surrounding matrix and vice versa, cells are constantly modifying their surrounding matrix. This interaction plays a key role in many biological processes ranging from development to diseases. To unravel this close interplay between cell behavior and matrix, we have developed techniques that allow us to control the matrix properties on the one hand and mechanotransduction in the cell on the other hand. Matrix properties that we control include stiffness, matrix fiber density, but also alignment of matrix fibers using microfluidics. In order to control mechanotransduction in the cell, we have developed a photo-activatable YAP, which allows us to activate YAP, a key mechanotransduction protein, with light and thus spatio-temporal control. I will show the application of these techniques to biological systems revealing the influence of underlying cell-matrix interactions and implications for in-vitro models of diseases.
Prof. Janos Vörös
Professor at ETH Zürich, Switzerland
Biosensors and Bioelectronics
János Vörös has studied Physics at the Eötvös Loránd University in Budapest. After receiving a diploma in Physics in 1995, he was a doctoral student at the Department of Biological Physics of the Eötvös University (in collaboration with Microvacuum Ltd.) where he received his PhD in Biophysics in 2000. Since 1998 he was a member of the BioInterface group in the Laboratory for Surface Science and Technology at the Department of Materials of ETH Zurich as visiting scientist, postdoc, and from 2004 as group leader of the Dynamic BioInterfaces group until 2006.
Prof. Vörös is interested in research and teaching in the areas of Biosensors, Bioelectronics, and Bottom-up Neuroscience. His research group focuses on the development of novel biosensor techniques for diagnostics and drug discovery; and on interacting with well-defined neural networks.
Keynote Talk (Oct. 8th, 4.15 pm): Bottom-up neuroscience – an interesting, easy, well-defined, reproducible, cheap and useful approach
Diseases of the human nervous system are one of the leading cause of death, scary and often painful. Unfortunately, it is very difficult to find new drugs for these diseases, because preclinical drug testing on animals have little predictive value on human safety/toxicity and especially on efficacy. Recent progress in human induced pluripotent stem cell (iPSC) technology in combination with emerging microphysiological systems (MPS) provides an opportunity to address some of the challenges related to investigating the nervous system and its diseases. This talk will introduce new tools to create and to interact with well-defined neuronal networks in vitro. For example, asymmetric PDMS microchannels can be used to guide the axonal growth in the desired direction on top of microelectrode arrays1-3 while nanochannels enable the tuning of the connectivity between pre- and postsynaptic neurons.4,5 This allows studying fundamental neuroscience paradigms6 and enables the creation of arbitrary network architectures with real neurons, including human iPSC-derived cells towards personalized medicine.6
All of the presented approaches are “scalable” which means that it is relatively straightforward to adapt the presented experimental methodologies for other systems. Special focus will be given to the practical aspects of this new methodology that should make it attractive to both PhD students and their PIs.
1 Engineered biological neural networks on high density CMOS based microelectrode arrays; J. Duru, et al.; Frontiers in Neuroscience, 2022.
2 Investigation of the input-output relationship of engineered neural networks using high-density microelectrode arrays; J. Duru et al., Biosensors and Bioelectronics; 2023 DOI: 10.1016/j.bios.2023.115591
3 Topologically controlled circuits of human iPSC-derived neurons for electrophysiological recordings; S. Girardin, et al.; Lab-on-a-chip, 2022.
4 Nanoscale patterning of in vitro neuronal circuits; J.C. Mateus and S. Weaver, et al; ACS-Nano, 2022.
5 Engineered biological neural networks as basic logic operators; J. Küchler, K. Vulić, et al.; Frontiers in Computational Neuroscience, 2025 DOI:10.3389/fncom.2025.1559936
6 An integrated in vitro platform and biophysical modeling approach for studying synaptic transmission in isolated neuronal pairs; G. Amos, et al. 2025 DOI: 10.1101/2025.06.04.657933
7 An in vitro platform for characterizing axonal electrophysiology of individual human iPSC-derived nociceptors. B.F. Clément, et al., Biosensors and Bioelectronics 2025 DOI: 10.1101/2025.01.08.631429
Prof. Chase Beisel
Professor at Botnar Institute of Immune Engineering, Basel, Switzerland
RNA Synthetic Biology
Chase Beisel is a Faculty Professor at the Botnar Institute of Immune Engineering. Prior to joining the BIIE, Chase was a tenured associate professor in the Department of Chemical & Biomolecular Engineering at North Carolina State University and a Full Professor and Head of Department at the Helmholtz Institute for RNA-based Infection Research, with a joint appointment in the Medical Faculty of the University of Würzburg. As part of the BIIE, Chase leads a research group devoted to bacterial immune engineering. His group aims to understand the functional diversity of immune systems bacteria employ to counter infections by bacteriophages and other mobile genetic elements and to translate discoveries into new innovations to improve the health and wellbeing of children and adolescents globally. His group’s work has driven seminal discoveries within CRISPR-Cas immune systems along with the development of diverse CRISPR-based technologies, which led to multiple awarded patents and two start-up companies (Locus Biosciences, Leopard Biosciences) Chase co-founded.
Keynote Talk (Oct. 9th, 9.15 am): CRISPR conflicts
CRISPR is best known as a revolutionary tool for genome editing, yet its origins lie in versatile adaptive immune systems in bacteria and archaea. While CRISPR can effectively memorize prior infections as genetically encoded guide RNAs that direct the immune system to the same sequence, bacteriophages have developed a collection of countermeasures to block CRISPR and proceed with the infection unabated. In this talk, I’ll describe my group’s work uncovering not only the diverse immune mechanisms employed by CRISPR but also how bacteriophages fight back, with an eye toward harnessing each new discovery to further expand and control the incredible suite of CRISPR technologies.
Prof. Olivia Merkel
Professor at LMU Munich, Germany
Smart drug delivery systems
Olivia Merkel has been a Professor of Drug Delivery at LMU Munich since 2015, Chair since 2022, and Co-Director of the Department of Pharmacy since 2024. She is a Registered Pharmacist, received a MS (2006) and a PhD (2009) in Pharmaceutical Technology as well as numerous awards, including an ERC Starting Grant, ERC Proof-of-Concept Grant and ERC Consolidator Grant, the APV Research Award and the Carl-Wilhelm-Scheele-Award. Merkel is the co-spokesperson for the BMBF Future Cluster CNAT-M and an author of over 120 articles and book chapters. She served as NIH reviewer from 2014-2015, SNSF reviewer from 2018-2022, is an Editorial Board member for JCR, EJPB, Molecular Pharmaceutics and other journals, Associate Editor for DDTR and WIREs Nanomedicine and Nanobiotechnology, was the President of the German Controlled Release Society in 2020 and the Chair of the CRS Focus Group on Transdermal and Mucosal Delivery from 2020-2022, and currently is a scientific advisory board member of Coriolis Pharma, AMW, and Corden Pharma as well as a co-founder of RNhale. Her research focuses mainly on RNA formulation and pulmonary delivery for the treatment of a variety of lung diseases.
Keynote Talk (Oct 9th, 1.00 pm): Polyspermines for therapeutic pulmonary RNA delivery
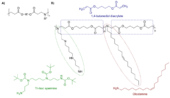
Delivery is the major hurdle thwarting the therapeutic potential of RNA medicines. While all siRNA drugs on the market target the liver, the lung offers a variety of currently undruggable targets which could be treated with RNA therapeutics. Hence, my lab rationally designs inhalable and biocompatible nanocarriers for efficient siRNA delivery to the lung. Poly(beta-amino ester)s (Fig. 1) are biodegradable cationic polymers capable of promoting nucleic acid delivery in vitro and in vivo and can be tailormade to investigate structure–function relationships. While biomaterials are commonly synthesized in large libraries and optimized empirically via one-variable-at-a-time experimentation, we combine Design-of-Experiments (DoE) with Molecular Dynamics Simulations and Machine Learning (ML) to accelerate the discovery and optimization process of polycationic siRNA nanocarriers at reduced wet-lab resources. Sophisticated in vitro and ex vivo models of the lung are available to screen therapeutic efficacy against respiratory viral infections, against transcription factors upregulated in asthma and COPD or against mutated oncogenes in the presence of relevant biologic barriers. Our previous results highlight oleylamine-modified spermine-based poly(β-amino ester)s (PBAEs) that efficiently encapsulate siRNA into nanoparticles <100 nm for up to 95% gene silencing in vitro. PBAE-based polyspermines successfully deliver siRNA for gene silencing in 2D cultures, Transwell® air-liquid-interface cultures, and in vivo. To compare polymer backbones, polyacrylamide (PAA)-based polyspermines were synthesized and resulted in more efficient siRNA delivery and gene silencing in Transwell® air-liquid-interface cultures compared with Lipofectamine but had a much more favorable safety profile in vitro and in vivo. After intratracheal administration to mice, the PAA-based polyplexes were efficiently taken up by Type II pneumocytes and successfully evaded recognition by macrophages in the lung. Additional in vivo experiments in models of disease are currently under way. Based on our preliminary data, polyspermines seem to be very promising candidates for RNA formulation and delivery with an excellent safety profile in the lung.
Prof. Henriette Uhlenhaut
Professor at TUM & Director at Helmholtz Zentrum, Munich, Germany

Metabolic Programming - Nuclear Hormone Receptors in Health and Disease
Prof. Uhlenhaut's (*1977) research focuses on investigating gene regulatory mechanisms that mediate hormone responses. Latest genome-wide techniques are combined with preclinical models to find new therapeutic approaches for metabolic or inflammatory diseases (e.g. diabetes, asthma). Prof. Uhlenhaut studied at the Technical University of Braunschweig and at Georgia Tech in Atlanta. She received her doctorate from EMBL in Heidelberg and, after postdoctoral research at the Salk Institute in San Diego and the MDC in Berlin, came to Helmholtz Zentrum Munich as group leader. She was also professor at the Gene Center of the LMU. In 2019, Prof. Uhlenhaut was appointed professor of 'Metabolic Programming' at the TUM. Since 2021, she has also been leading the Insitute for Diabetes and Endocrinology at Helmholtz Zentrum Munich as its director.
Keynote Talk (Oct 9th, 3.30 pm): Transcriptional control of hepatic metabolism by nuclear hormone receptors
The liver is a central organ controlling carbohydrate, fat, cholesterol and protein metabolism. With the ever-rising incidence of obesity and overnutrition, the prevalence of liver diseases such as MALFD, MASH, fibrosis and liver cancer is dramatically increasing everywhere, with few drugs to combat their pathogenesis. Nuclear hormone receptors such as PPARs, FXR, LXR, GR and other steroid receptors pose promising drug targets due to their inherent ability to regulate gene expression in response to ligand binding. Nuclear receptors act as transcription factors to control essential cellular functions such as inflammation, differentiation, proliferation, metabolism and circadian rhythms. Here our latest research on genome-wide hormone receptor binding, coregulator recruitment, chromatin dynamics, transcriptomics, proteomics and metabolomics will be presented to showcase how nuclear receptors regulate homeostasis in mice and humans.